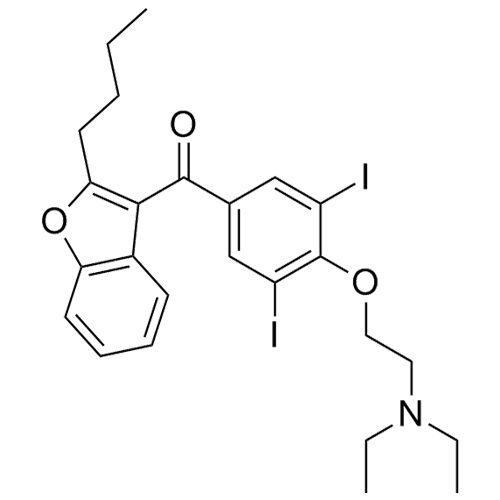
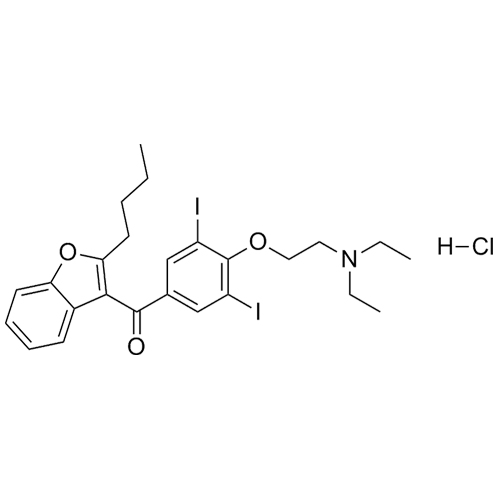
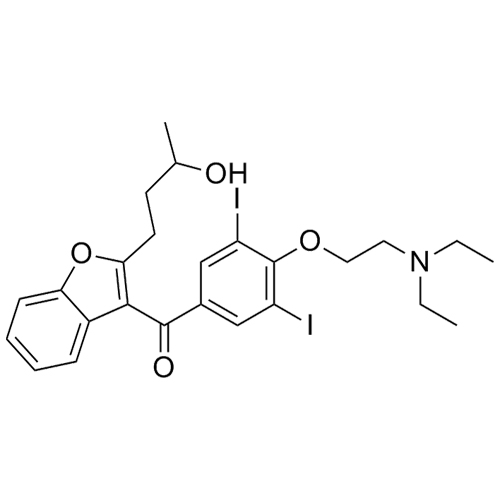
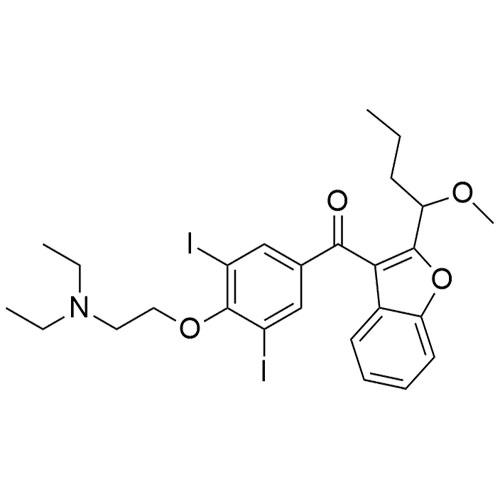
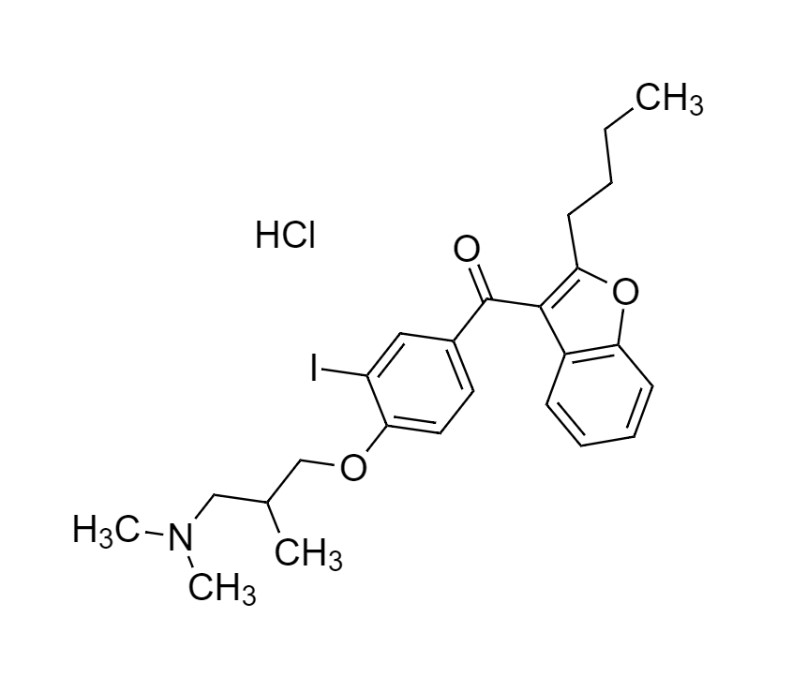
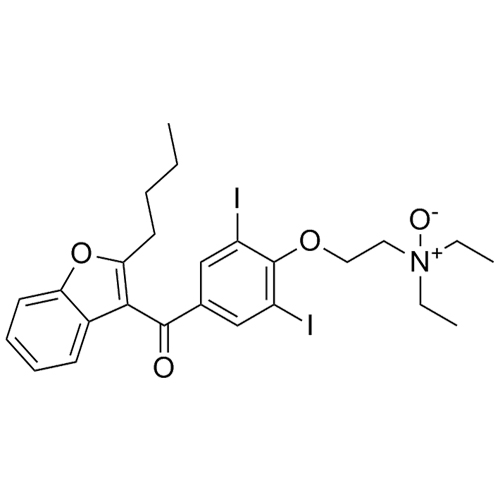
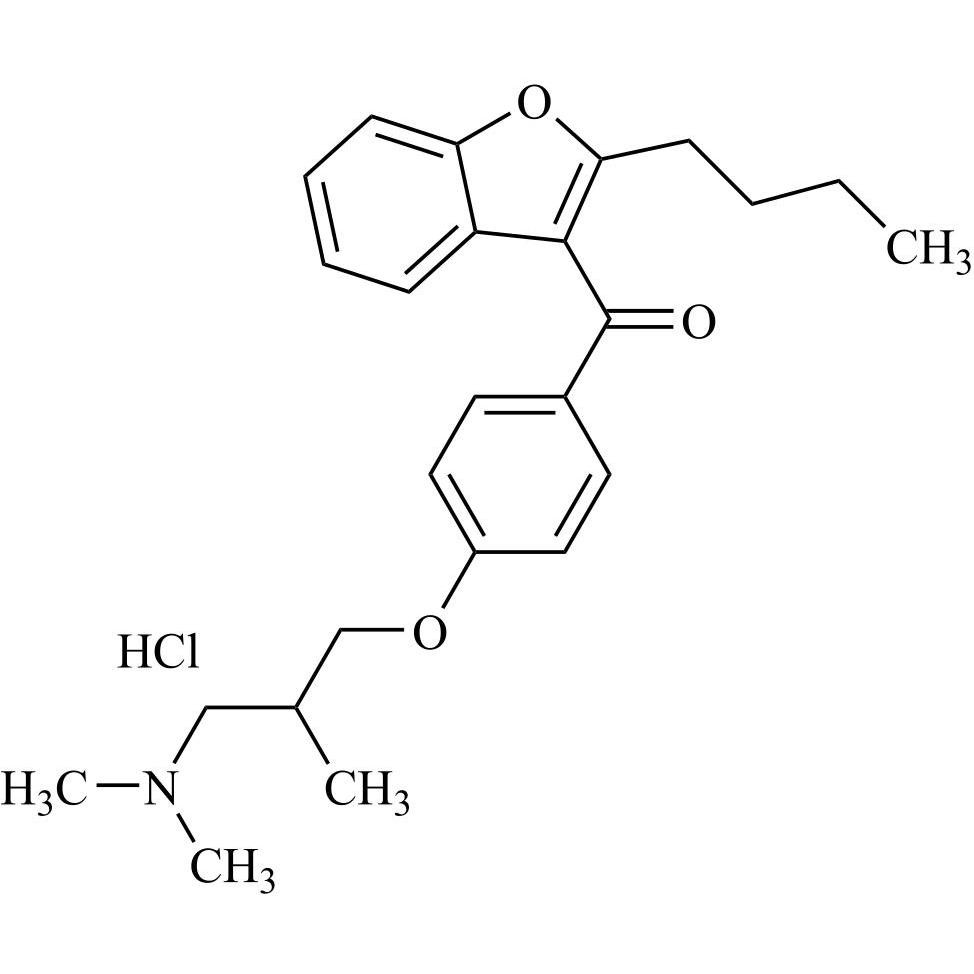
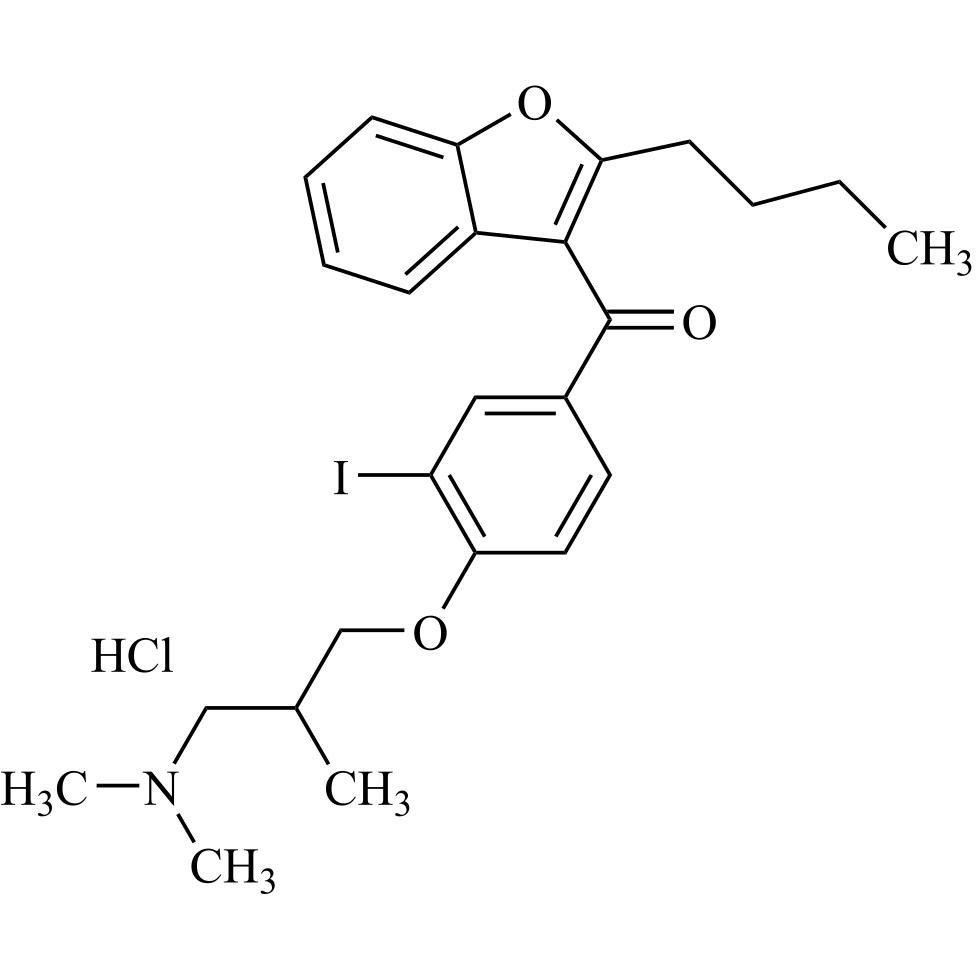
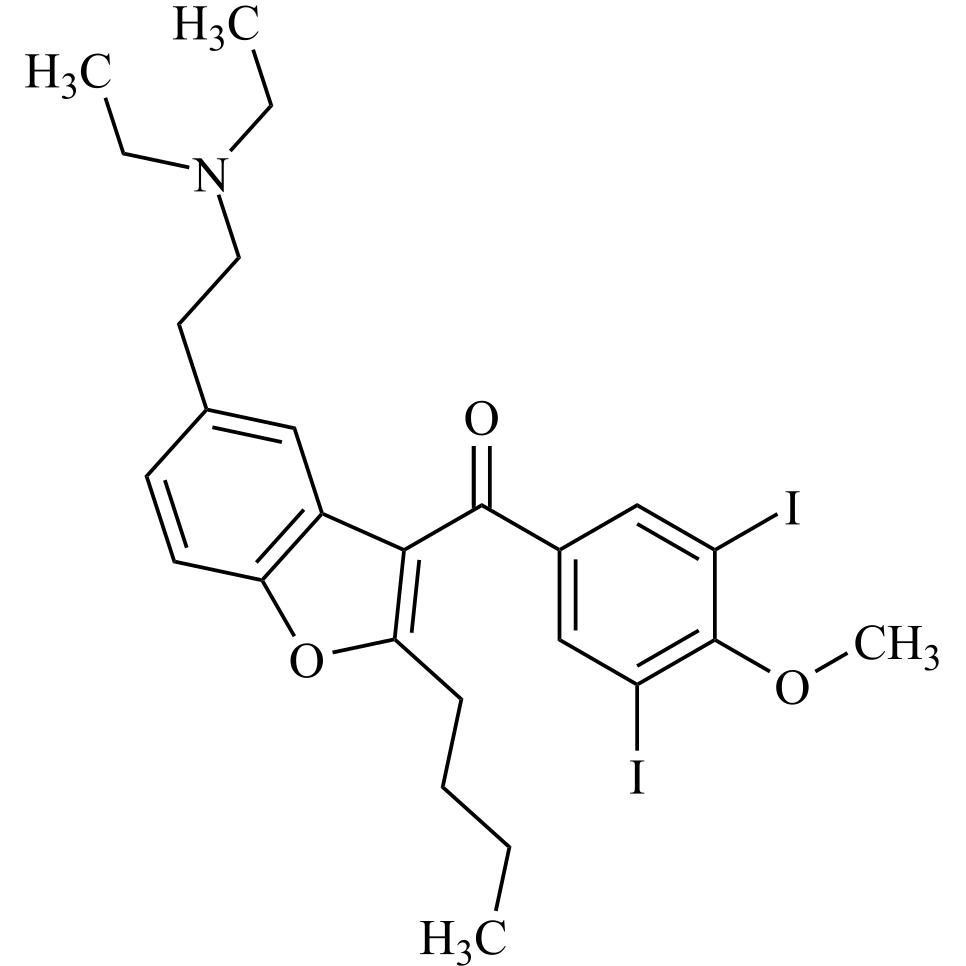
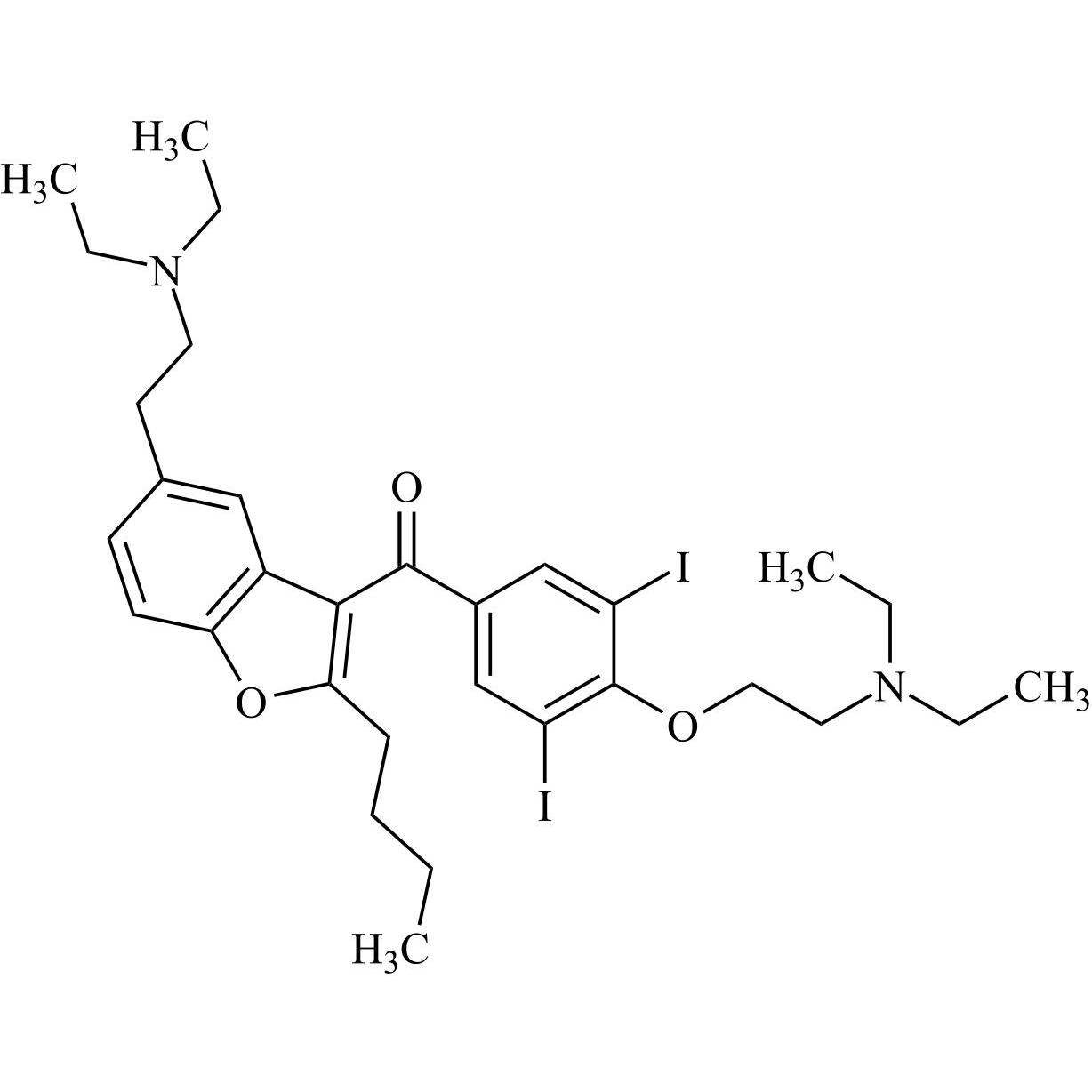
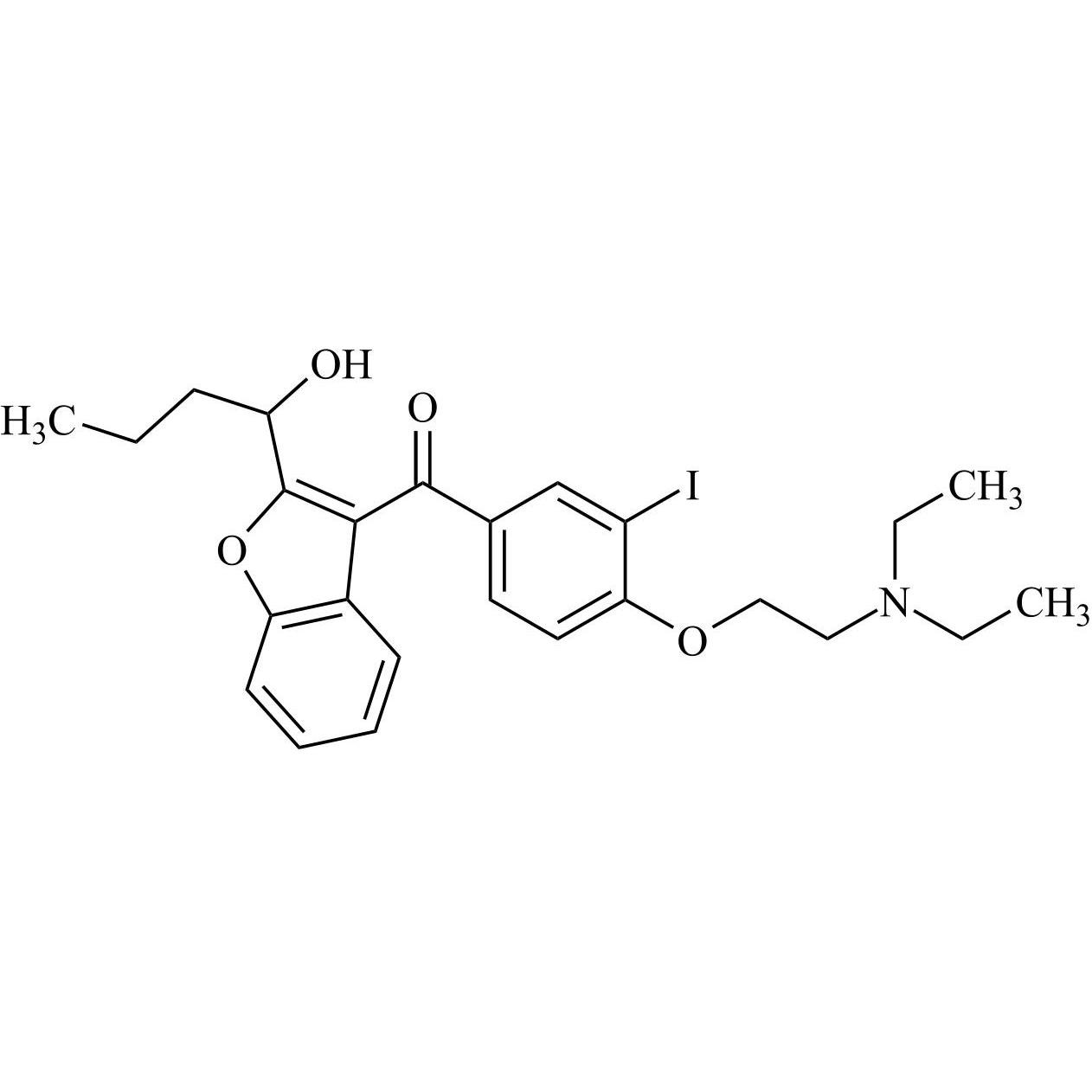
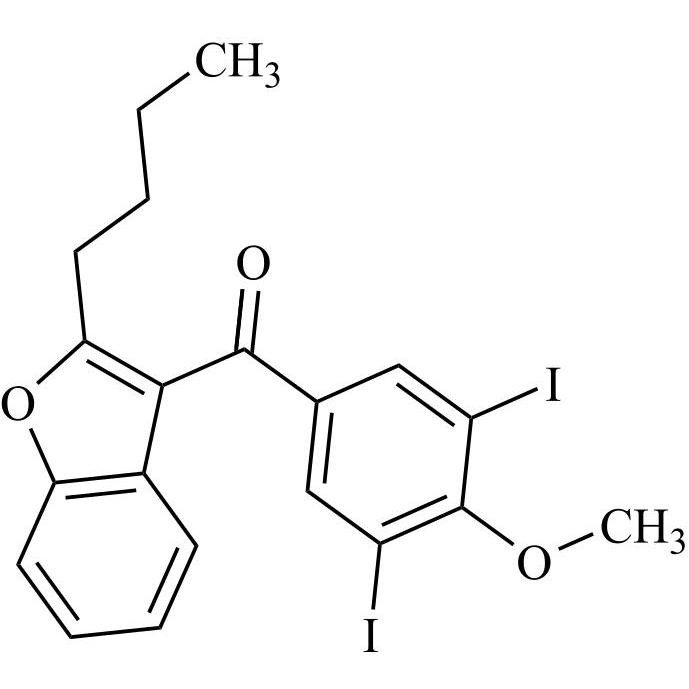
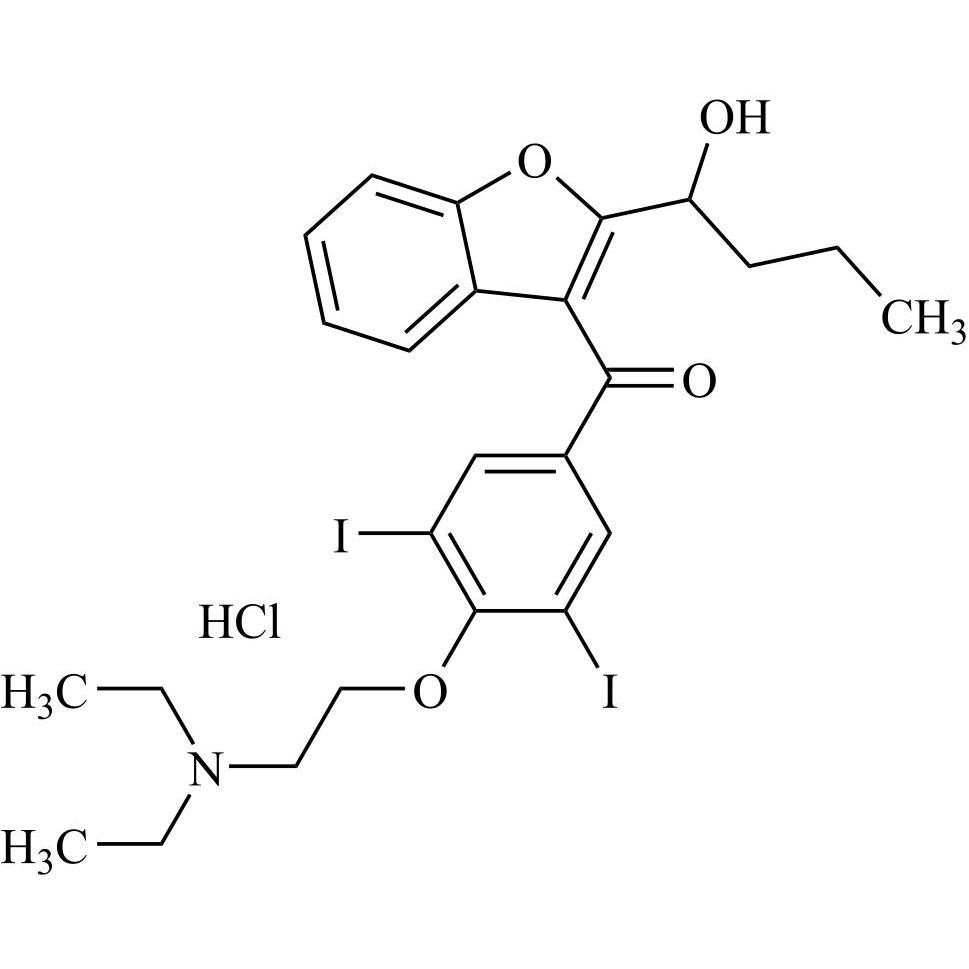
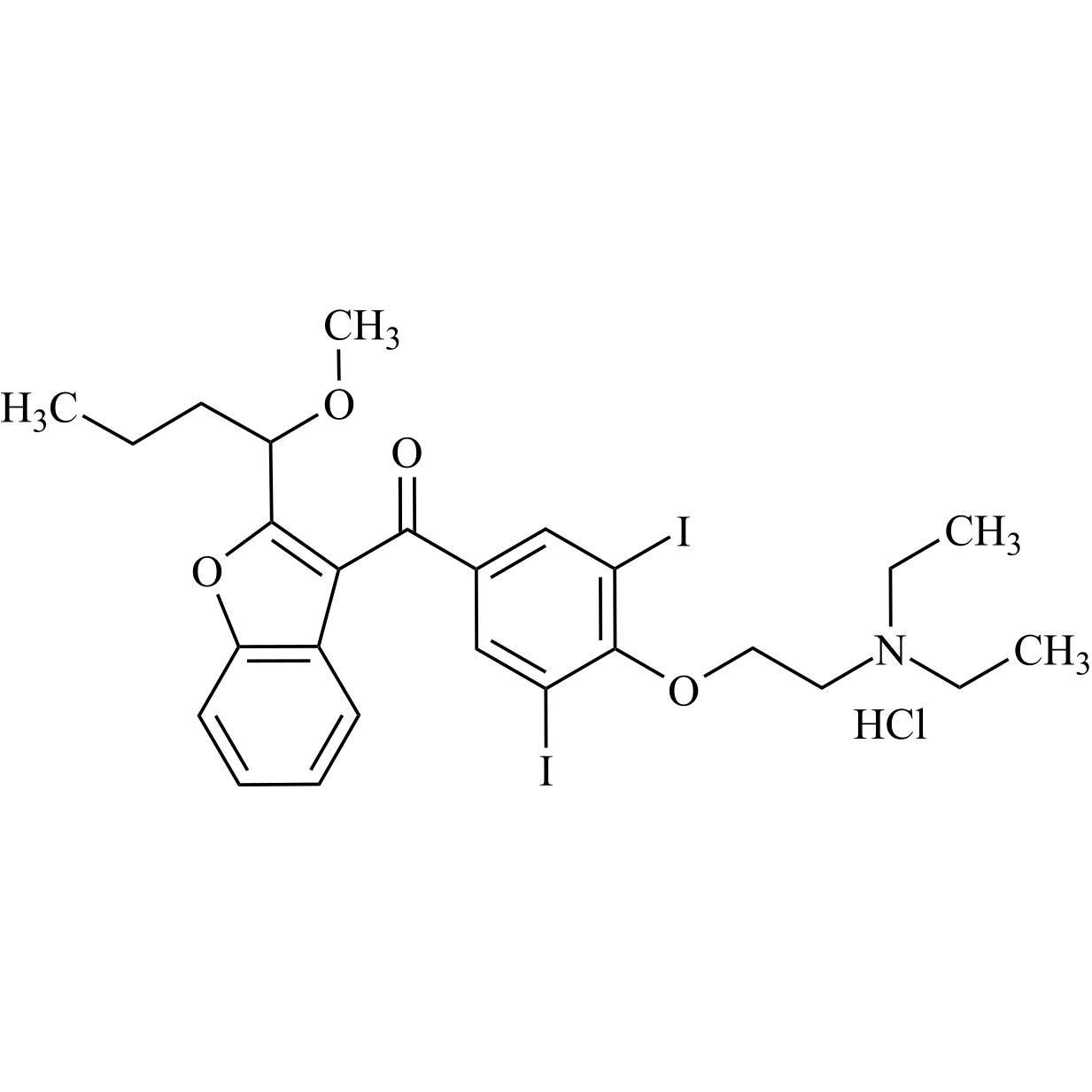
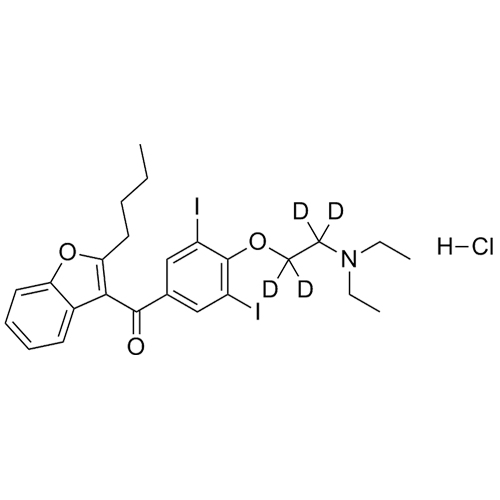
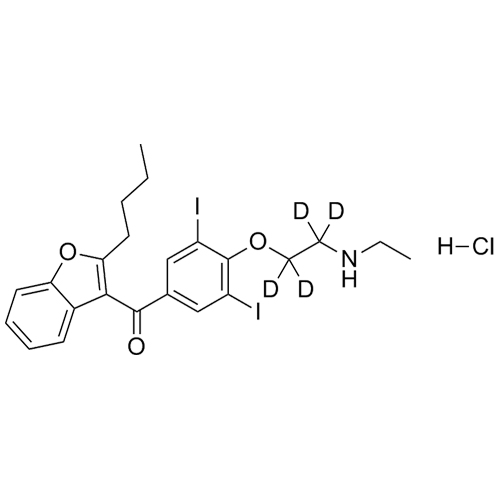
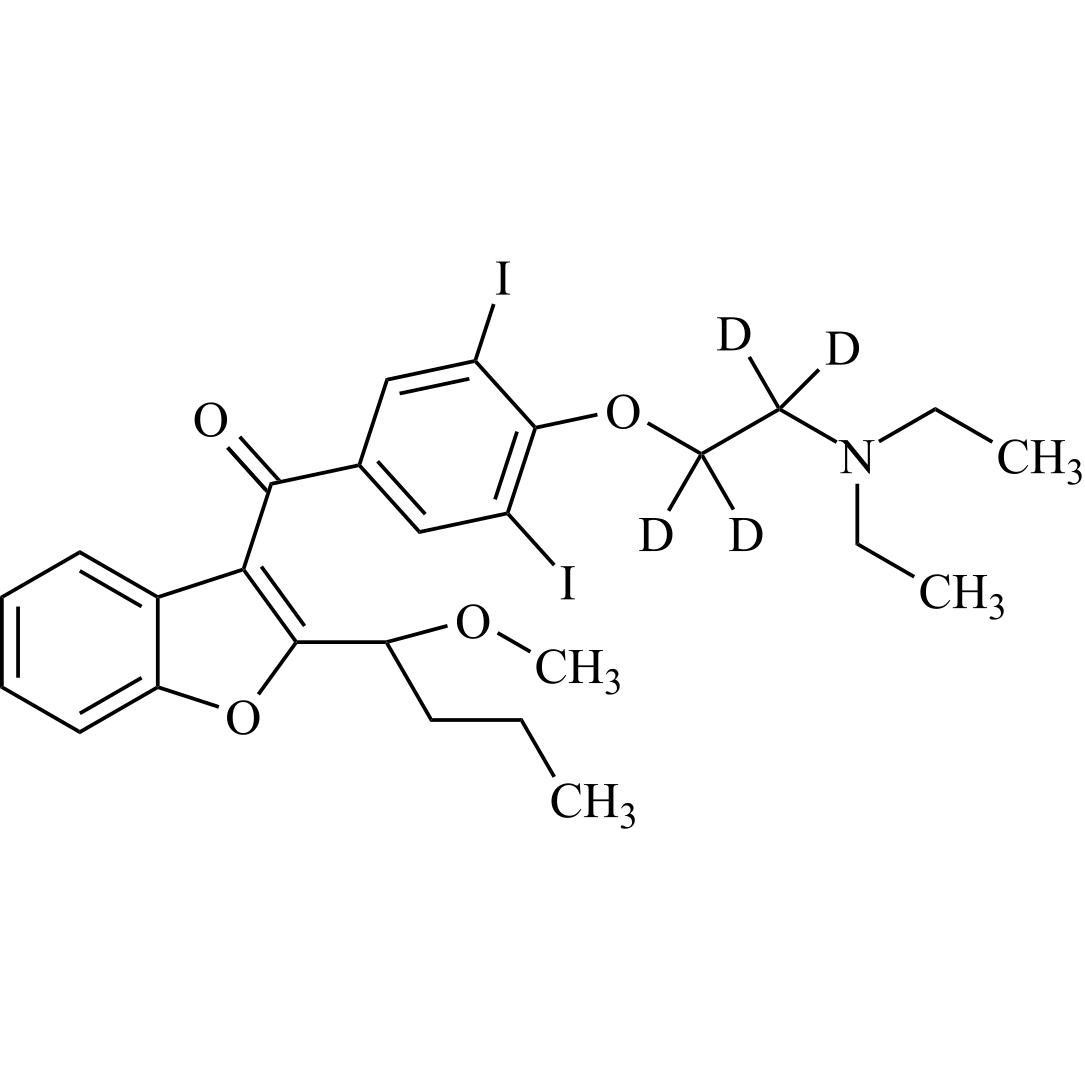
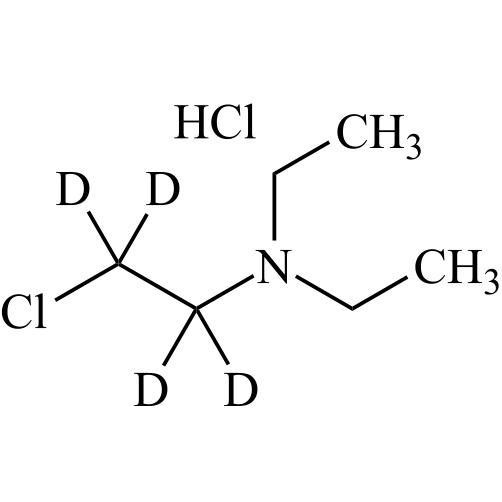
- Synonyms(4-(2-(diethylamino)ethoxy)-3,5-diiodophenyl)(2-(1-methoxybutyl)benzofuran-3-yl)methanone hydrochloride; 1-Methoxy Amiodarone; [4-[2-(Diethylamino)ethoxy]-3,5-diiodophenyl][2-(1-methoxybutyl)-3-benzofuranyl]methanone; [2-[(1RS)-1-Methoxybutyl]benzofuran-3-yl][4-[2-(diethylamino)ethoxy]-3,5-diiodo...
- Description
(4-(2-(diethylamino)ethoxy)-3,5-diiodophenyl)(2-(1-methoxybutyl)benzofuran-3-yl)methanone hydrochloride; 1-Methoxy Amiodarone; [4-[2-(Diethylamino)ethoxy]-3,5-diiodophenyl][2-(1-methoxybutyl)-3-benzofuranyl]methanone; [2-[(1RS)-1-Methoxybutyl]benzofuran-3-yl][4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl]methanone; Amiodarone EP Impurity G ; Amiodarone USP Related Compound G
Amiodarone EP Impurity G HCl is a fully characterized chemical compound used as a reference standard of API Amiodarone. The standard offered is compliant with regulatory guidelines. Amiodarone EP Impurity G HCl is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - NA
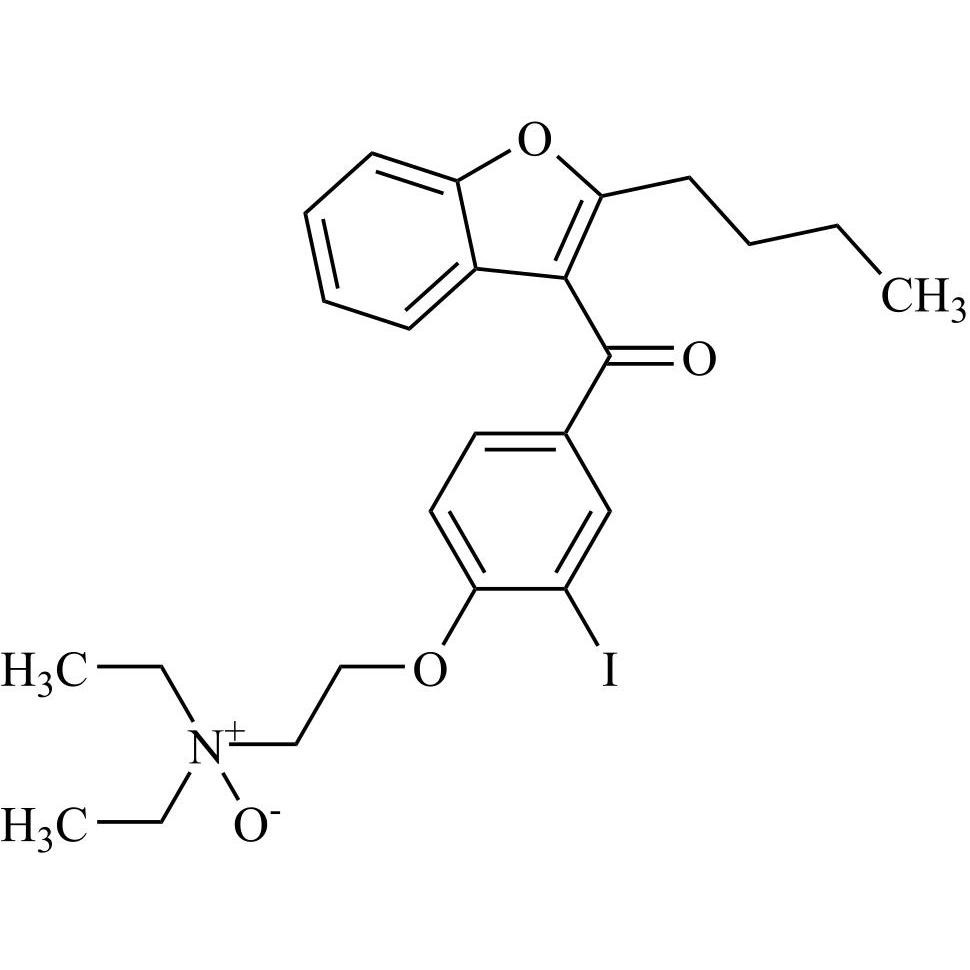
Related products
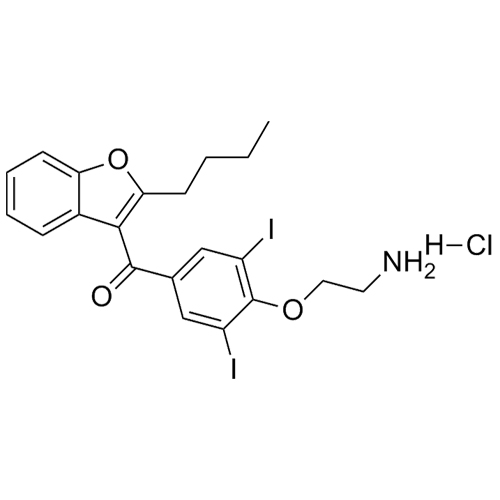
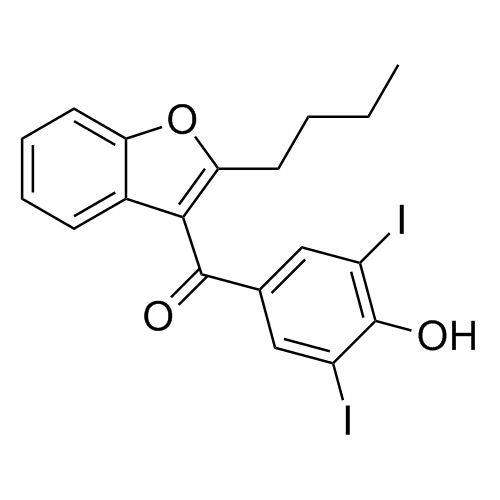
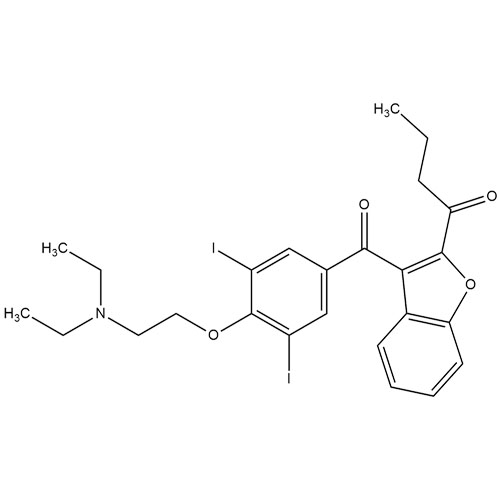
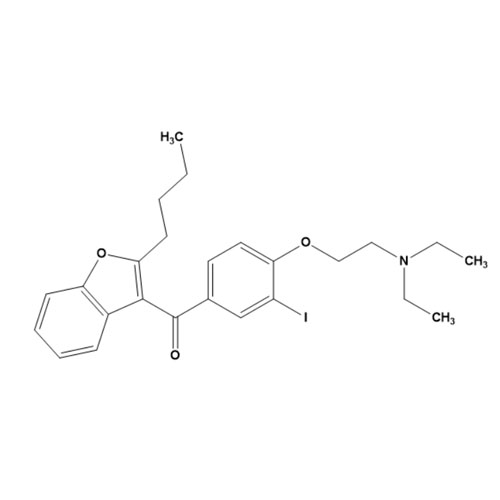
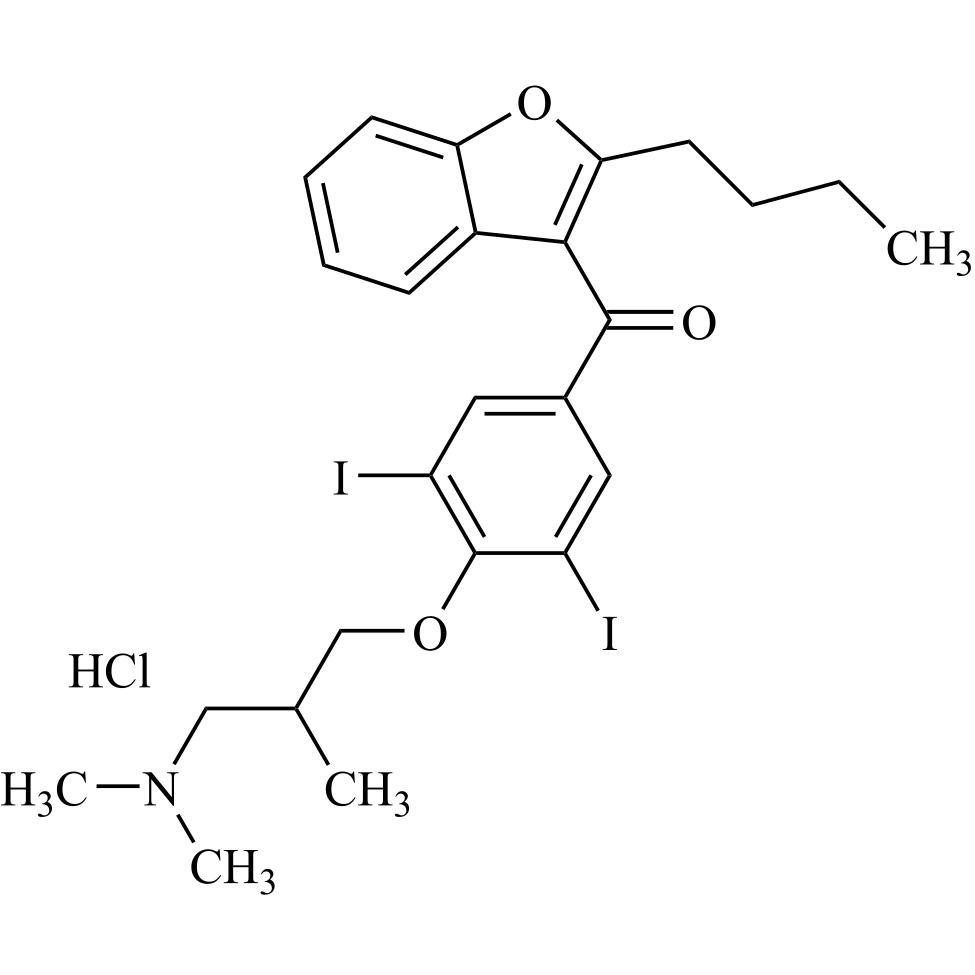
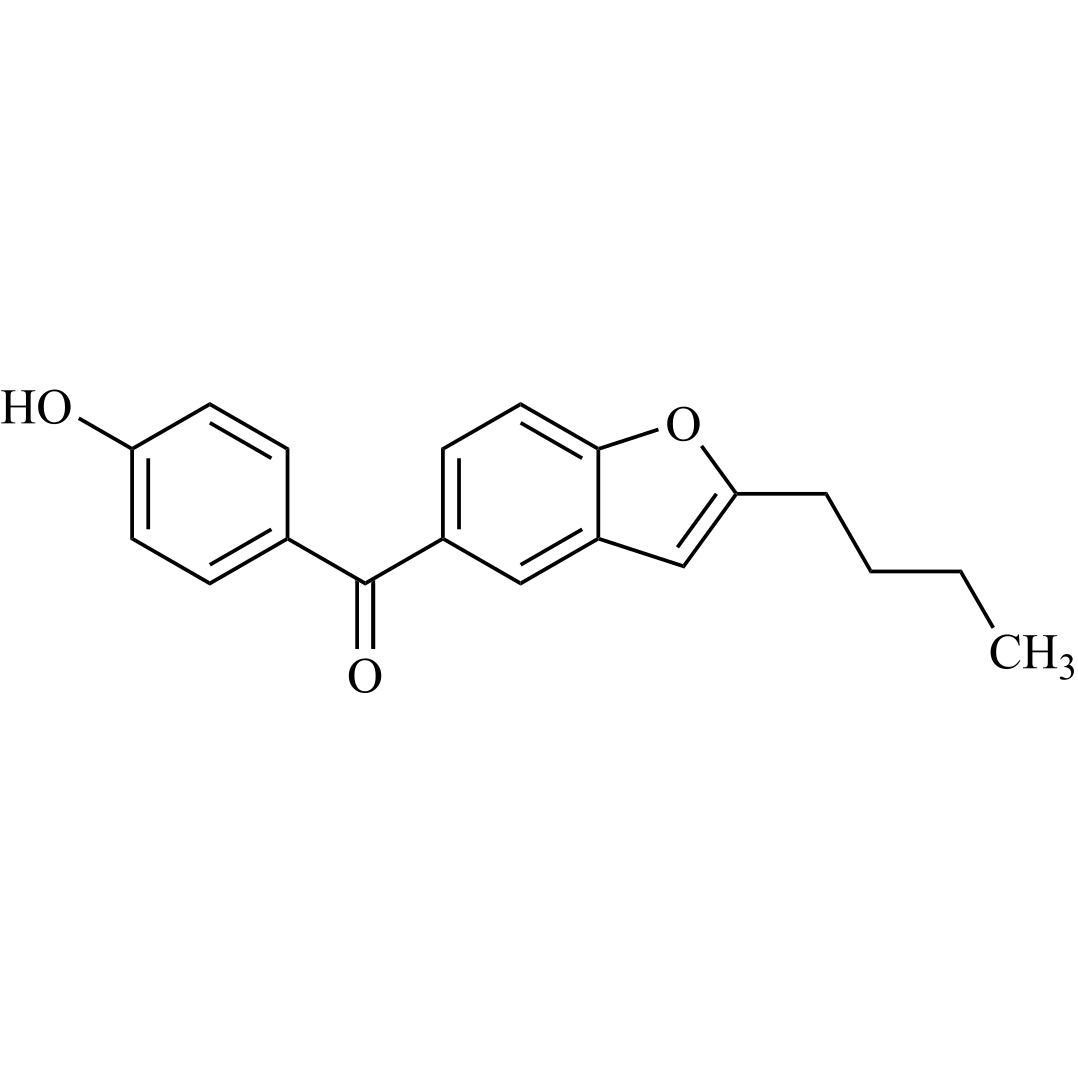
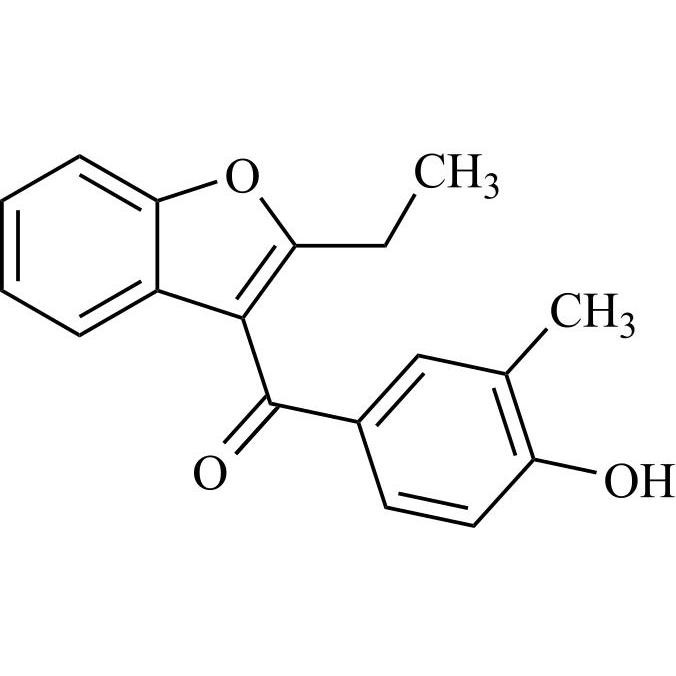
Amiodarone EP Impurity C HCl (Deiodo Impurity)

M.F.
M.W. 519.42; 36.46
CAT# AR-A01940
CAS# 1397201-93-2
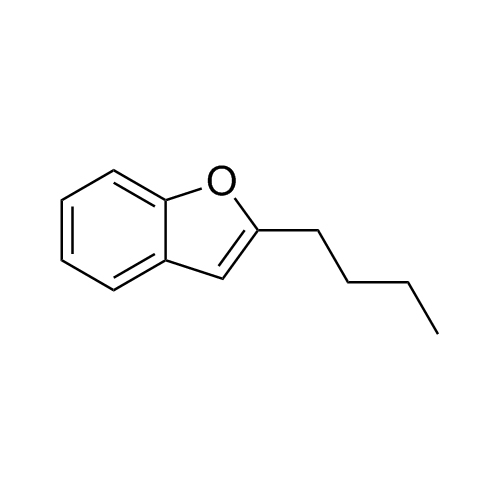
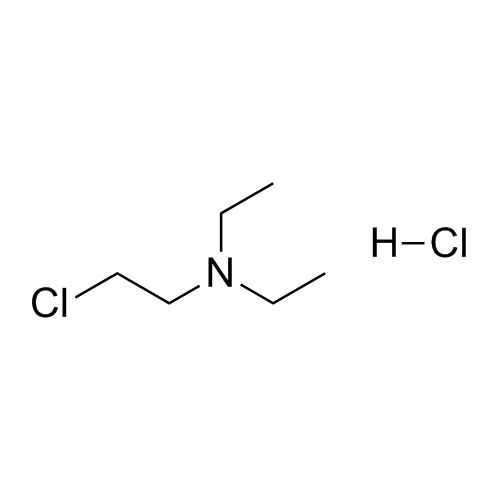
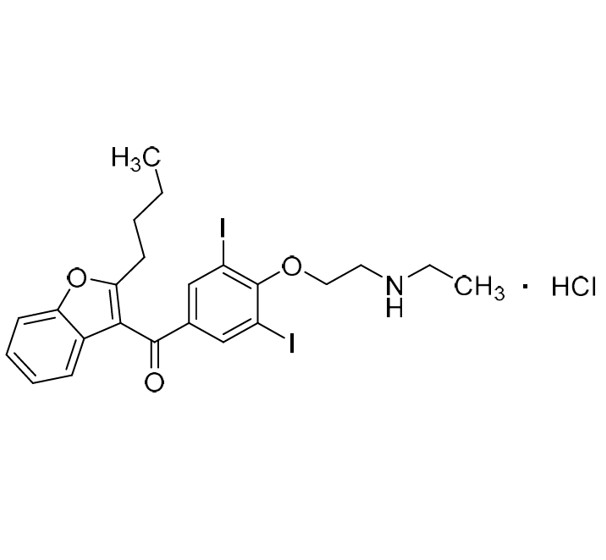
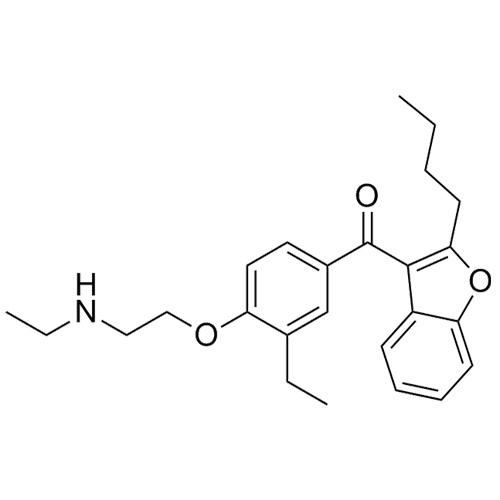
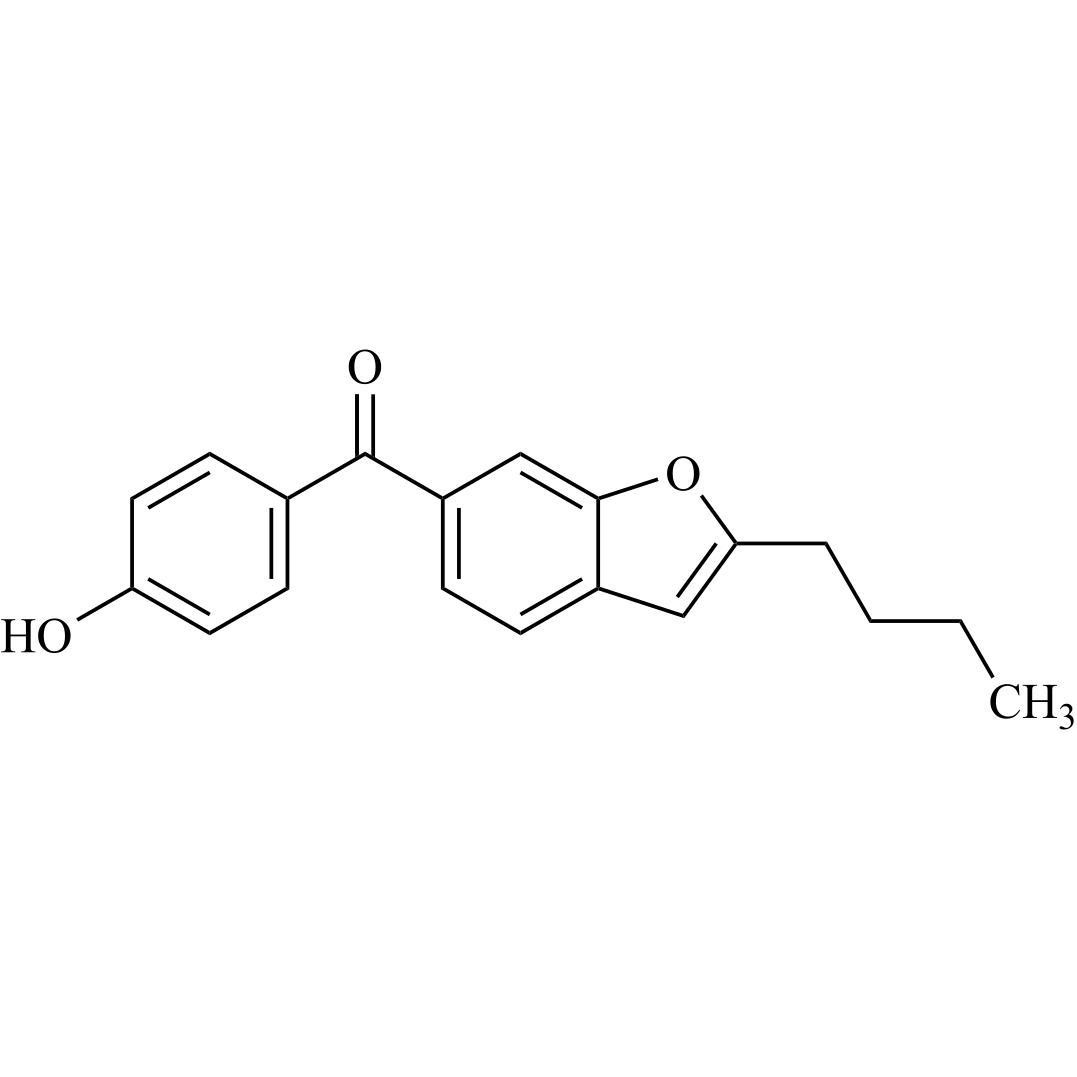
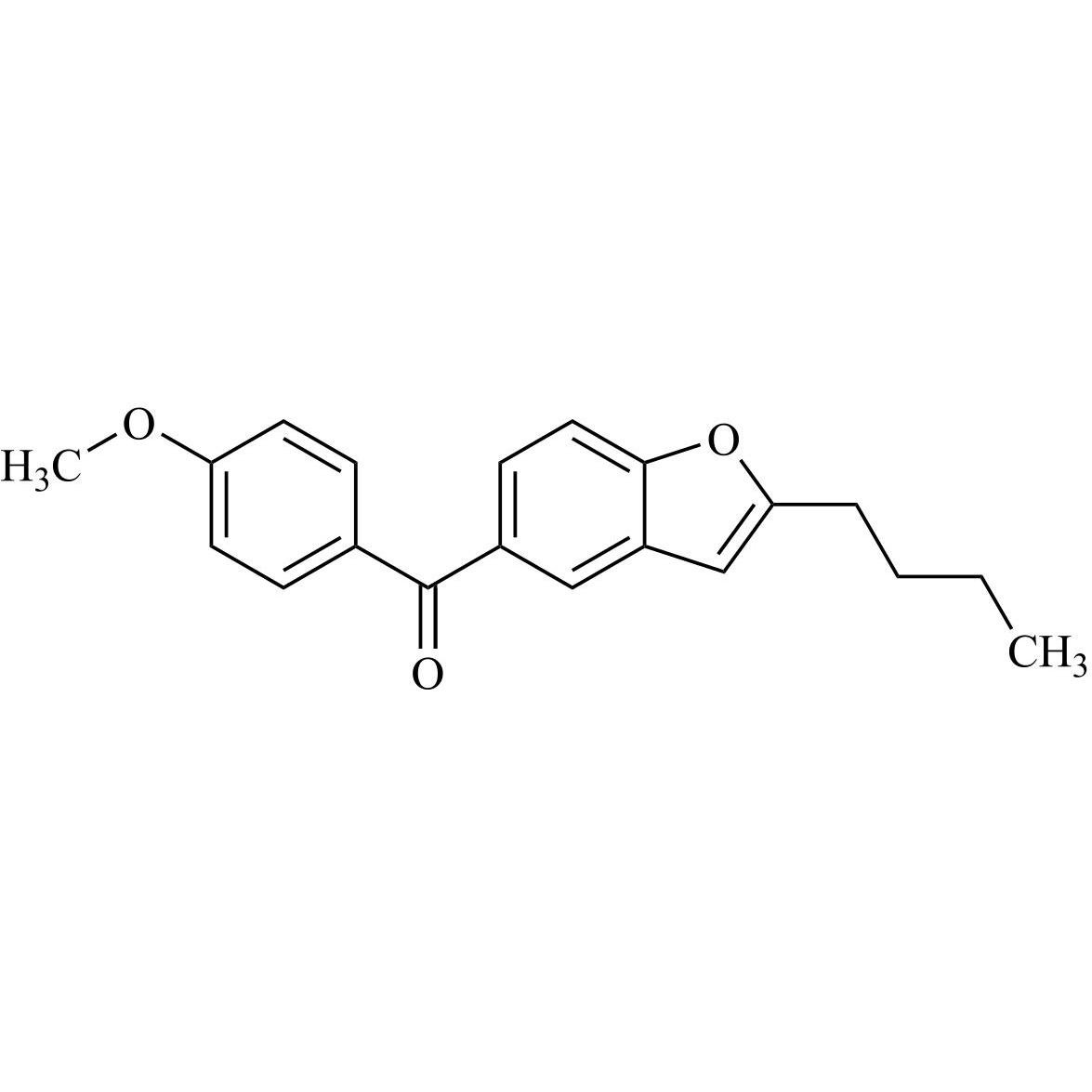
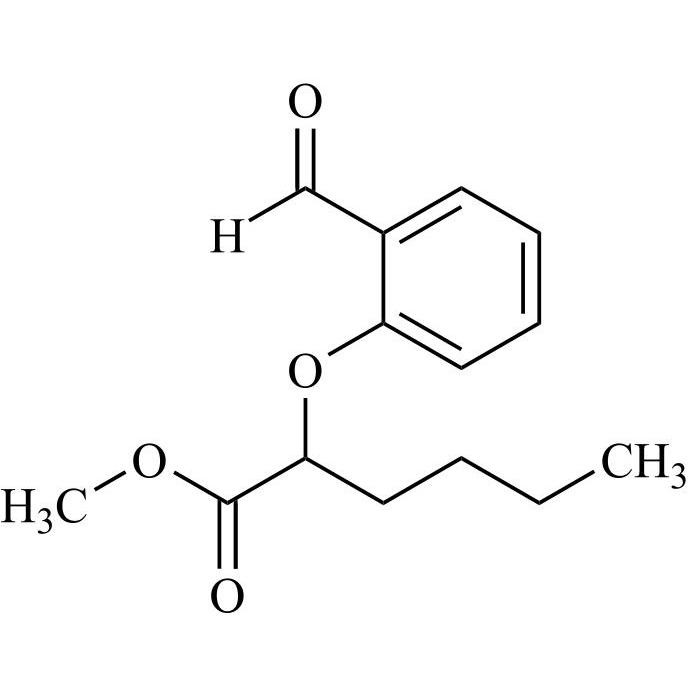
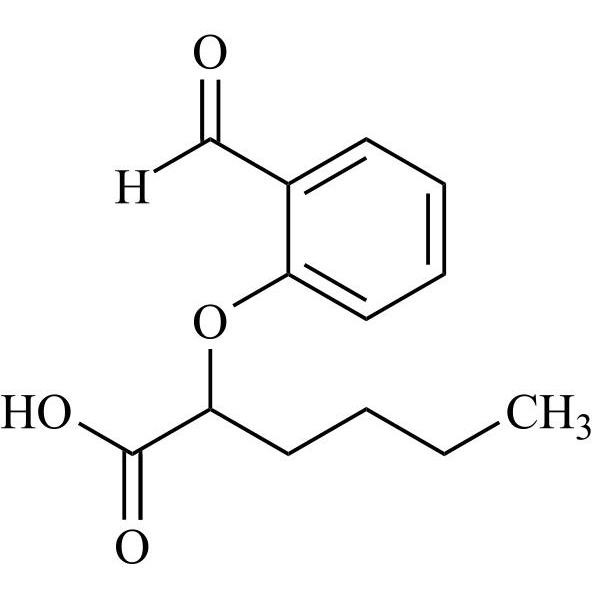
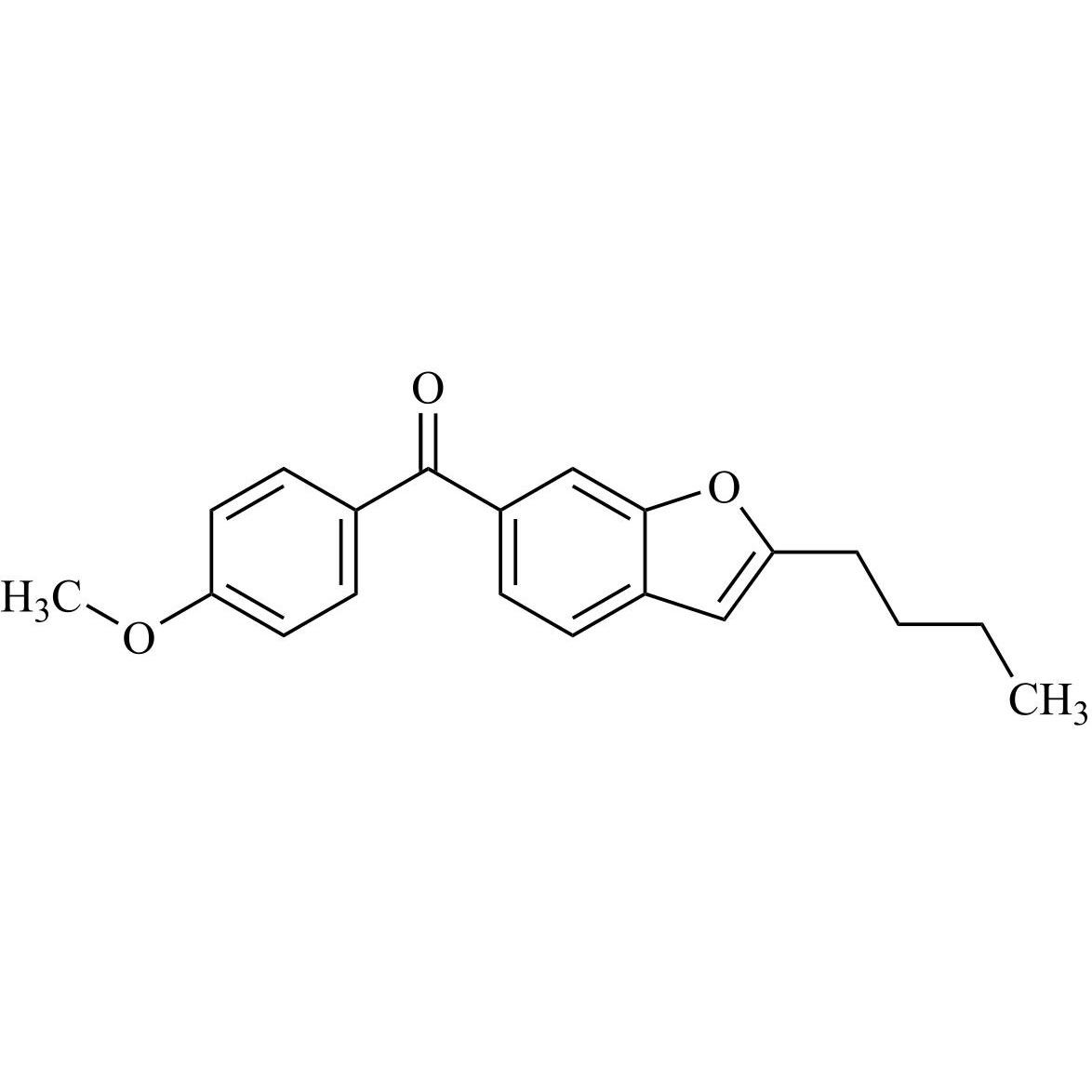
Des-O-[2-(diethylamino)ethyl]-1-methoxy Amiodarone
![Show details for Des-O-[2-(diethylamino)ethyl]-1-methoxy Amiodarone Picture of Des-O-[2-(diethylamino)ethyl]-1-methoxy Amiodarone](https://bc3c83a0874864a50753-7010fd7f2bdf81ab41ee28479491701e.ssl.cf2.rackcdn.com/media//catalog/AR-A06279.jpg?size=256)
M.F.
M.W. 576.17
CAT# AR-A06279
CAS# 1391054-75-3